The Science Behind the Technology

True liposomes must meet four requirements in order to provide maximum effectiveness. Specnova is the only company to scientifically validate all four liposome requirements.
 The size of the liposome must be between 100-200 nm
The size of the liposome must be between 100-200 nm
 The liposomes must have a strong encapsulation
The liposomes must have a strong encapsulation
 The liposome must be spherical in shape
The liposome must be spherical in shape
 They must be well dispersed and free of leakage
They must be well dispersed and free of leakage
Every liposomal ingredient requires a unique technique for its creation and scale-up to full manufacturing. Specnova tests every batch of our liposomal ingredients using our exclusive TruLiposome® 4-step Validation test. We verify the size, shape, dispersal, and stability of the liposomes before they are shipped to our customers. These tests are done with specialized equipment and are not available to all companies.
Specnova guarantees its LipoVantage® ingredients with TruLiposome® 4-Step Validation. This robust set of testing methods accurately validates the four liposomal requirements, ensuring maximum effectiveness.
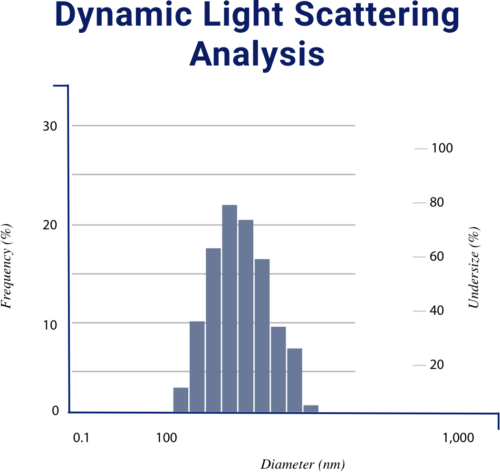

Dynamic Light Scattering Analysis (DLS) is conducted to identify the average size in nanometers of the liposome as well as to indicate the stability of the liposome due to increased surface-to-volume ratio. LipoVantage® liposomes are around 175.3 nm in size. The decrease in particle size allows for an increase in bioavailability and results in a more stable colloidal suspension.
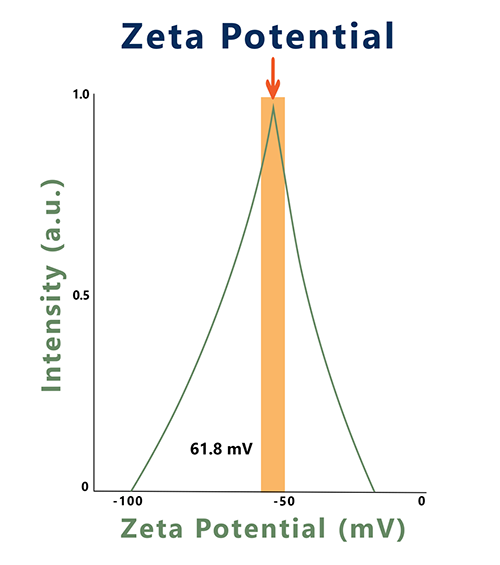

Zeta potential values are studied and measured to confirm a strong encapsulation of the active ingredient and stable colloidal suspension. This results in protection against the degradation of the active component in the gastrointestinal tract and thereby results in a more bioavailable, active component.


LipoVantage® liposomes are shown to be spherical in shape using Scanning Electron Microscope (SEM) images. The image shows that the liposomes are spherical.
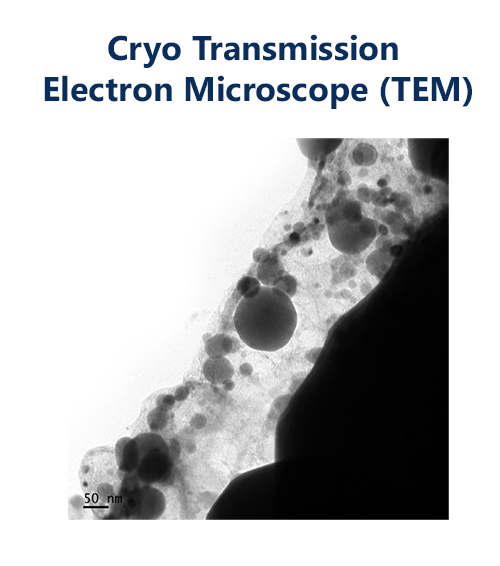

LipoVantage® ingredients are shown to be stable using Cryo Transmission Electron Microscope (TEM) images. The image shows that the liposomes are spherical in shape, well dispersed, and there is minimal liposomal debris which might indicate vesical rupture. The core of the liposomal is well defined by the dark portion of the structure.
DualHydrogel® Technology
Better Liposomal Protection
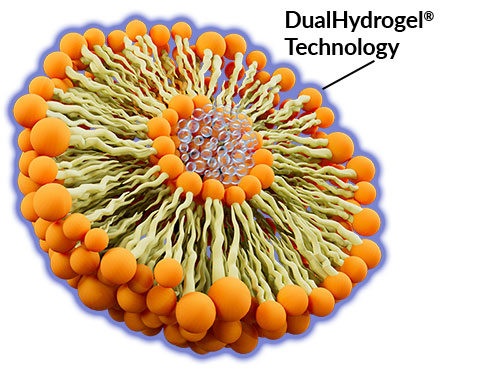
DualHydrogel® Technology helps set LipoVantage® apart from the competition through its ability to:
-
Protect the liposome
-
Increase stability in the body
-
Increase bioavailability
-
Increase shelf life